heat of hydrogenation depends on|heat of hydrogenation vs combustion : Manila 1. "Heat of hydrogenation" is a subset of "heat of reaction" which is nothing but the standard enthalpy change ( ΔH∘ Δ H ∘) in the system as reactants are converted . Lista dos melhores filmes de drama turcos selecionados pelos visitantes do nosso site: Love Likes Coincidence, Cinco Graças, Kördüğüm, Fatmagül: A Força do Amor, Ezel, Kocan Kadar Konus 2: Dirilis, A Garota Chamada Feriha, Sono de Inverno, Terrorismo em Nova York, Conquest 1453. No topo, há novos filmes de 2021, uma descrição da trama .
0 · lowest heat of hydrogenation dienes
1 · highest heat of hydrogenation
2 · heat of hydrogenation vs combustion
3 · heat of hydrogenation proportional to
4 · decreasing order of hydrogenation
5 · decreasing order of heat hydrogenation
6 · correct order of heat hydrogenation
7 · More
8 · 1 butyne heat of hydrogenation
WEBAssista vídeos pornô de Rabuda Dancando de graça, aqui no Pornhub.com. Descubra a crescente coleção de vídeos e filmes Mais relevantes explícitos em alta qualidade. Nenhum outro site pornô é mais popular e tem mais cenas de Rabuda Dancando do que o Pornhub! Navegue pela nossa incrível seleção de de vídeos pornô em HD em qualquer .
heat of hydrogenation depends on*******The experimental values of ΔH0 Δ H 0 for hydrogenation of a number of alkenes and alkynes are listed in Table 11-2. The ΔH0 Δ H 0 calculated from average bond energies .
1. "Heat of hydrogenation" is a subset of "heat of reaction" which is nothing but the standard enthalpy change ( ΔH∘ Δ H ∘) in the system as reactants are converted .Solution. The heat of hydrogenation is the measure of the stability of an alkene. Lower the heat of hydrogenation of alkene the more stable it is and vice versa. Alkyl substitution .
heat of hydrogenation depends on Measurement of the heat evolved in the hydrogenation of alkenes gives information as to the relative stabilities of alkenes, provided that the differences in \(\Delta S^0\) values are small. The experimental .Heat of hydrogenation vs stability of alkenes. In this video, we see how we can figure out the stability of different alkenes from their heat of hydrogenation. We make a conclusion - "higher the stability of alkene implies lower heat of hydrogenation" Created by .Definition. The heat of hydrogenation is the amount of energy released when a double bond in an alkene reacts with hydrogen gas to form a single bond, turning it into an . Ethene + H 2 Pd/C −. −. → Ethane. The heat of whatever event at constant pressure, qp, is simply the enthalpy of such an event, ΔH. For a hydrogenation . Example: Hydrogenation of isomeric alkene,1-butene,cis-2-butene, and trans-2butene all these three add one molecule of hydrogen to produce the same .
Heat of Hydrogenation of Dienes. Consider dienes with the molecular formula, C 5 H 8, giving rise to two isomeric diene one is an isolated diene, 1,4-pentadiene, and another isomer is a conjugated diene, 1,3 .
Study Notes. The two alkenes, cis-CH 3 CH=CHCH 3 and (CH 3) 2 C=CH 2 have similar heats of hydrogenation (−120 kJ/mol and −119 kJ/mol, respectively), and are therefore of similar stability. However, they are .heat of hydrogenation vs combustion via GIPHY. So what we’re really saying here is that alkene stability increases with increasing substitution of hydrogen for carbon. [The image above uses heat of hydrogenation data for the series hex-1 . Measurement of the heat evolved in the hydrogenation of alkenes gives information as to the relative stabilities of alkenes, provided that the differences in \(\Delta S^0\) values are small. The experimental . Heat of hydration is defined as the amount of energy released when one mole of ions undergo hydration. It is also termed as hydration enthalpy. It is the standard enthalpy of catalytic hydrogenation of alkenes. The heat of hydrogenation depends on the stability of alkenes. Stability of alkenes indicates that they do not convert into alkanes.
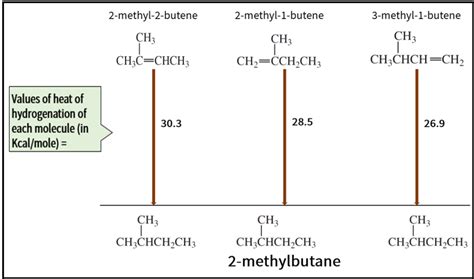
This heat of reaction can be used to evaluate the thermodynamic stability of alkenes having different numbers of alkyl substituents on the double bond. For example, the following table lists the heats of hydrogenation for three C 5 H 10 alkenes which give the same alkane product (2-methylbutane). Since a large heat of reaction indicates a high .This is the enthalpy change that occurs during catalytic hydrogenation. It is used to compare the stability of pi-bonded molecules, and as a probe of alkene stability. Order of enthalpy of hydrogenation is reverse of stability of alkenes. I I is conjugated and most substituted, thus evolves the least heat, followed by I V. Ethene + H 2 Pd/C −. −. → Ethane. The heat of whatever event at constant pressure, qp, is simply the enthalpy of such an event, ΔH. For a hydrogenation reaction, the enthalpy of hydrogenation is simply the enthalpy of reaction, or ΔH rxn. This enthalpy could be broken down into which bonds were broken or made. One might call those ΔH . Note: We can also predict the heat of combustion using the primary, secondary, and tertiary hydrogens. The carbon bond with primary hydrogen would be stronger when compared to carbon bond with secondary hydrogen, and carbon bond with tertiary hydrogen. If the number of primary hydrogen is more, then the heat of .Heat of Hydrogenation. The heat of hydrogenation of an unsaturated compound is the enthalpy of reaction between a hydrogen molecule and the compound. It is a measure of the stability of carbon-carbon double and triple bonds. Small absolute values denote a stable bond [5]. Examples: The heat of hydrogenation of alkene is -30 kCal mol-1, and that .Bond strength depends on the efficiency with which orbitals can overlap. In general, s orbitals overlap more efficiently than do p orbitals; . Figure 7.6.3: Trans-2-butene is the most stable because it has the lowest heat of hydrogenation. Note. In cycloalkenes smaller than cyclooctene, the cis isomers are more stable than the trans as a .
Heats of Hydrogenation of Bicyclo[2.1.0]pent-2-ene, an Antiaromatic System The heat of hydrogenation of the olefinic double bond in bicyclo[2.1.0]pent-2-ene (2) and in the 5,5-dimethyl derivative . 1. "Heat of hydrogenation" is a subset of "heat of reaction" which is nothing but the standard enthalpy change ( ΔH∘ Δ H ∘) in the system as reactants are converted to products. In catalytic hydrogenation, as hydrogen molecules are added to atoms bearing double/triple bonds, the enthalpy change which accompanies the reaction is called as .

2. Catalytic hydrogenation (Sections 6.1–6.3) Alkenes react with hydrogen in the presence of a platinum, palladium, rhodium, or nickel catalyst to form the corresponding alkane. Addition of hydrogen halides (Sections 6.4–6.7) A proton and a halogen add to the double bond of an alkene to yield an alkyl halide.
Escape! That's the motto in our free Escape games. You are .
heat of hydrogenation depends on|heat of hydrogenation vs combustion